Breast cancer was first described in the Edwin Smith Egyptian papyrus, around 3000 B.C.E. – 1500 B.C.E. Hippocrates described stages of breast cancer in 400 B.C. William Halsted has been credited with popularizing the modified radical mastectomy (MRM) around 1882. In the 1970s, Umberto Veronesi challenged the use of modified radical mastectomy over BCT by leading randomized clinical trials. Bernard Fisher constructed a randomized trial that was published in 1977 comparing different surgical techniques for breast cancer which proved to be incredibly influential to the acceptance of breast-conserving therapy.
Etiology
- Genetic mutations
- Risk factors
- High risk
- BRCA gene with family history of breast cancer
- > 20% lifetime risk based on family history (e.g., ≥ 2 primary relatives with premenopausal-onset, bilateral cancer)
- DCIS (ipsilateral breast at risk) and LCIS (both brests have same high risk)
- Atypical hyperplasia
- Prior breast cancer
- Chest radiation between ages 10 – 30 years old
- Li-Fraumeni, Cowden/PTEN, Bannayan-Riley-Ruvalcaba syndrome
- Genetic mutations: ATM, BARD1, BRIP1, CHD1, CHEK2, NBN, NF1, PALB2, PTEN, RAD51C, RADS1D, STK11, TP53
- Moderate risk
- First-degree relative with breast cancer
- Age > 35 years old at first birth
- Low risk
- Early menarche
- Late menopause
- Nulliparity
- Proliferative benign disease
- Obesity
- Alcohol use
- Hormone replacement therapy
- High risk
Epidemiology
- Second leading cause of cancer-related deaths (1st = lung cancer)
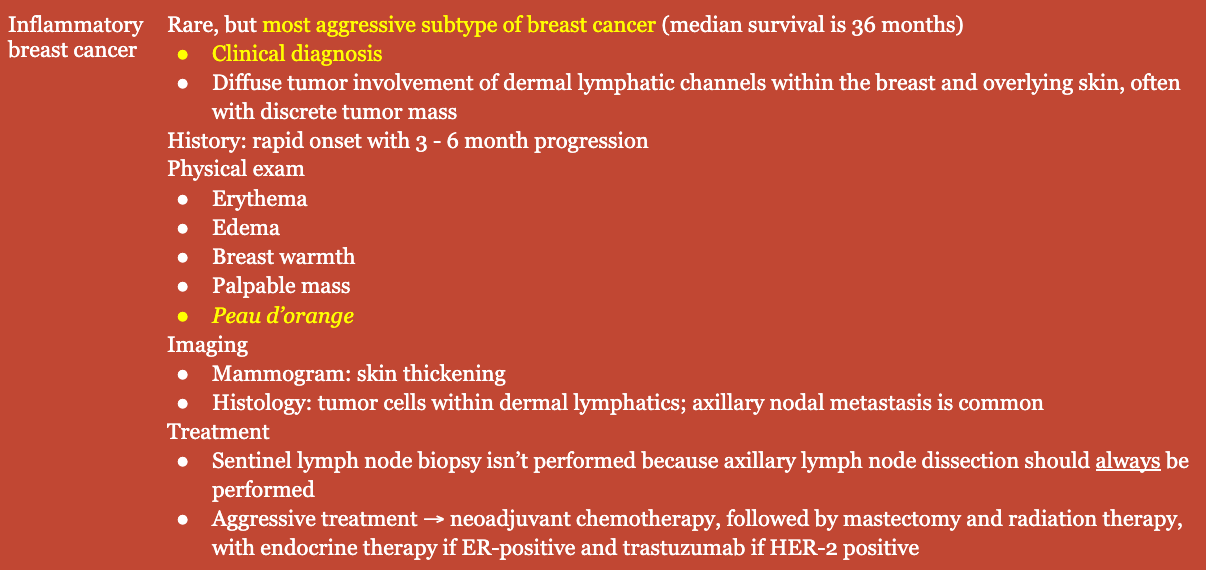
History
- Constitutional symptoms
- Bone pain
- Weight loss
- Respiratory changes
- ± Rapid onset
Physical Exam
- Breast retraction
- Nipple inversion/retraction
- Excoriation of the superficial epidermis
- Skin dimpling
- Peau d’orange
- Skin edema
- Palpable mass
Labs → genetic mutations
- BRCA1/BRCA2 (BReast CAncer gene): repairs damaged DNA
- PALB2 (Partner And Localizer of BRCA2): tumor suppressor gene
- CHEK2 (CHEckpoint Kinase 2): involved in DNA repair, cell cycle arrest or apoptosis in response to damaged DNA
- CDH1 (cadherin-1): role in epithelial cell adherence; associated with increased risk of invasive lobular breast cancer
- PTEN (Phosphatase and TENsin): tumor suppressor gene; associated with Cowden syndrome; cancer and tumors in breasts, digestive tract, thyroid, uterus, and ovaries
- STK11 (Serine/Threonine Kinase 11): tumor suppressor gene; associated with Peutz-Jeghers syndrome
- TP53: tumor suppressor gene; associated with Li-Fraumeni syndrome, leukemia, brain tumors, and sarcomas
Imaging
- Mammogram
- Detects masses ≥ 5 mm
- Screening mammograms
- USPSTF: biennial starting at age 40 years; continue until age 75 years
- ACOG: offer starting at age 40 years, recommend no later than age 50 years; repeat annual or biennial; continue until age 75 years
- ACS: offer annually from age 40 – 44 years; recommend annually from age 45 – 54 years; option to switch to biennial from age 55 and older (continue screening if woman is in good health and life expectancy of 10 years)
- Findings: irregular borders; spiculated; multiple clustered. small, thin, linear, crushed-like and/or branching calcifications; ductal asymmetry, distorted architecture
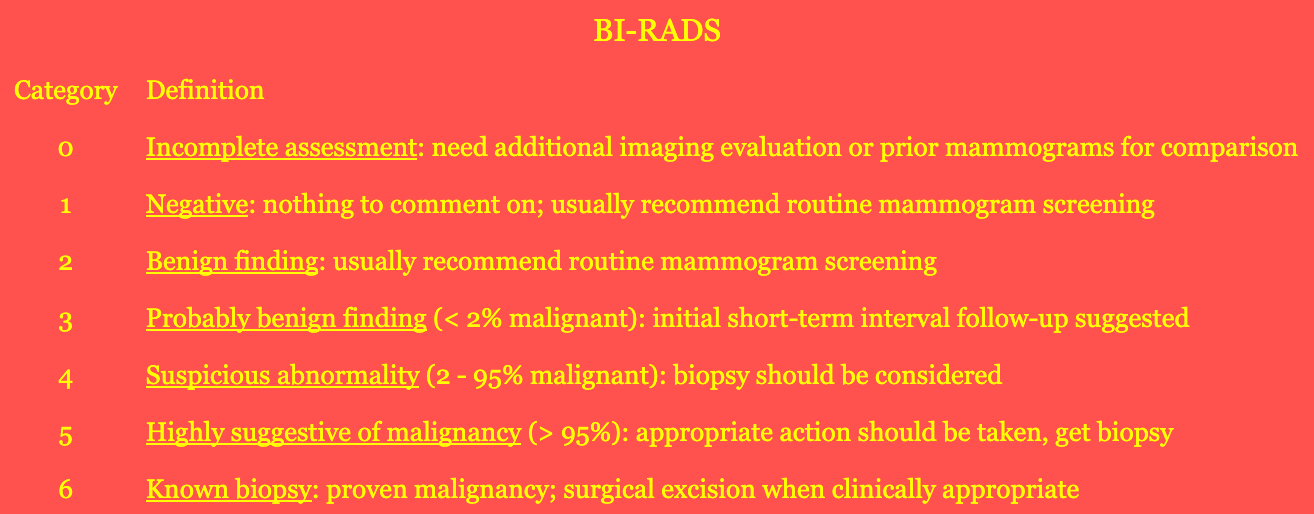
- Breast Imaging Reporting and Data System (BI-RADS): classifies mammogram findings
- Breast US findings: hypoechogenicity; internal calcifications; shadowing; lesion taller than it is wide; spiculated, indistinct, or angular margins
- Biopsy → determine tumor histology, grade, estrogen receptor (ER) and progesterone receptor (PR) status, and human epidermal growth factor receptor-2 (HER-2) status
- Core needle biopsy (CNB): preferred diagnostic method for palpable or image-detected lesions
- Open surgical biopsy: completed for lesions not amenable to CNB or cases where CNB is nondiagnostic
- Hormone receptors (ER, PR)
- Often positive in postmenopausal women
- Better prognosis if positive; best prognosis if ER-positive and PR-positive
- HER-2/neu receptor
- A receptor tyrosine kinase
- HER-2 positive is associated with worse prognosis
- CT, bone scans, and other imaging studies are reserved for patients with clinically positive nodes, abnormalities on CBCs, CXRs, and those with locally advanced or inflammatory breast cancer
Staging
- AJCC (American Joint Committee on Cancer) 8th edition is the most widely used system. It has clinical and pathologic staging systems.
- Clinical staging → physical exam and imaging
- Pathologic staging
- Histology and histologic grade
- Lymph node staging
- Sentinel lymph node biopsy (SLNB)
- Sentinel lymph node: first node draining area of primary breast tumor; most likely to contain metastatic disease
- Allows patients to avoid axillary lymph node dissection if sentinel nodes are negative
- Axillary lymph node dissection (ALND)
- Sentinel lymph node biopsy (SLNB)
- Other variables → ER, PR, HER-2 status, circulating tumor cells, disseminated tumor cells, multigene recurrence score, response to chemotherapy
Treatment
- Note
- Treatment of breast cancer is very complex
- Consult NCCN for most up-to-date guidelines
- Treatments as described on this page are as accurate as possible with relevant, approachable information for the student level
- Diagnosis of breast cancer is needed before definitive surgical treatment
- Absence of metastatic disease
- Early-stage breast cancer → surgical excision of tumor and surgical staging of regional lymph nodes
- Breast-conserving therapy
- Options
- Lumpectomy plus ALND or SLNB
- Quadrantectomy plus ALND or SLNB
- Eligibility
- Tumors that can be excised with clear margins and adequate cosmesis
- Negative margins (i.e., “no ink on tumor”)
- Histology: invasive lobular cancers and cancers with an extensive intraductal component (as long as clear margins can be obtained)
- Considerations
- Must be willing to complete postoperative radiation therapy and surveillance
- Feasibility of tumor size and breast size with adequate cosmesis
- Several contraindications
- Options
- Mastectomy
- Options
- Subcutaneous mastectomy (simple mastectomy)
- Leaves small amount of breast tissue and preserves nipple-areola complex
- Indications: DCIS, LCIS
- Contraindications: breast cancer
- Modified radical mastectomy (MRM)
- Removes all breast tissue, including nipple-areola complex
- Includes axillary node dissection (levels I and II)
- Subcutaneous mastectomy (simple mastectomy)
- Indications
- Tumors in patients with contraindications to radiation therapy
- Absolute contraindications: pregnancy
- Relative contraindications: systemic scleroderma, active SLE, prior radiation to breast/chest wall, severe pulmonary disease, severe cardiac disease (if left-sided tumor), inability to lie supine, inability to abduct arm on affected side, p53 mutation
- Multicentric tumors
- Tumors large to breast size
- Tumors with extensive calcifications on mammography
- Tumors in which clear margins cannot be obtained on wide local excision
- Tumors in patients with contraindications to radiation therapy
- Prophylactic mastectomy
- As opposed to follow-up ± tamoxifen/raloxifene
- Considerations
- BRCA gene carrier
- Non-BRCA gene carrier
- Family history, but patient not undergone genetic testing
- Family history, patient BRCA-negative, no known BRCA family member
- Family history or carrier of BRCA gene
- History of chest radiation before age 30 years
- Need one of following: high patient anxiety, poor patient access for follow-up exams and mammograms, lesions that are difficult to follow on exam or mammogram
- Options
- Radiation therapy
- Postoperative radiation for patients with breast-conserving therapy
- Chemotherapy
- Preoperative chemotherapy
- May help decrease tumor size to permit breast-conserving therapy in those who wouldn’t otherwise be a good candidate for breast-conserving therapy
- Considered for patients with large tumors who are recommended to have postoperative chemotherapy
- Preoperative chemotherapy
Relevant Information
- BRCA1 and BRCA2 mutations also increase risk of ovarian cancer. Hysterectomy and bilateral salpingo-oophorectomy may be considered in BRCA positive patients with a history of breast cancer.
- Triple-negative breast cancers: tumors that lack expression of ER (estrogen receptor), PR (progesterone receptor), and HER-2 (human epidermal growth factor receptor 2)
- Histologic subtypes of breast cancer have some relationship with prognosis, but there are many other factors at play as well




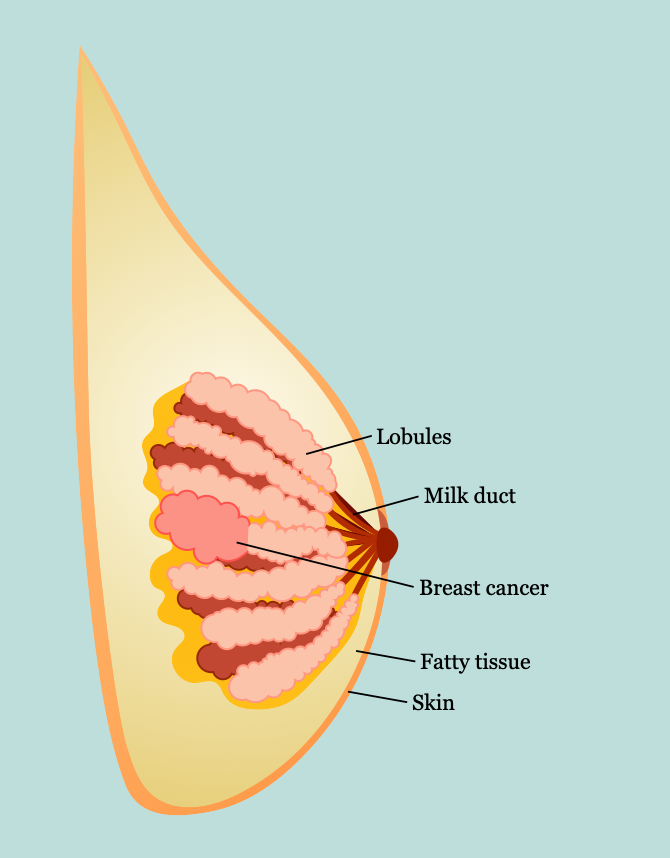
- Breast anatomy
- Lies between subdermal layer of adipose tissue and superficial pectoral fascia
- Cooper ligaments
- Provide structural support and shape (anchored into the skin)
- Infiltration by tumors can produce tethering, resulting in dimpling on the breast tissue
- Lymphatics
- Lymph nodes
- Level 1: located lateral to the lateral border of the pectoralis minor muscle
- Level II: located posterior to the pectoralis minor muscle as well as anterior to the pectoralis minor and posterior to the pectoralis major (Rotter or interpectoral nodes)
- Level III: located medial to pectoralis minor muscle and include subclavicular nodes
- Most drains to axillary nodes (97%)
- Any quadrant can drain into internal mammary nodes
- Supraclavicular nodes → N3 disease
- Primary axillary adenopathy → ≤ 1 is lymphoma
- Lymph nodes
- Nerves
- Long thoracic nerve
- Innervates serratus anterior muscle
- Injury → winged scapula
- Lateral thoracic artery supplies serratus anterior muscle
- Thoracodorsal nerve
- Innervates latissimus dorsi muscle
- Injury → weak arm pull-ups and adduction
- Thoracodorsal artery supplies latissimus dorsi
- Arises from posterior cord of brachial plexus; enters axillary space under axillary vein (close to long thoracic nerve)
- Medial pectoral nerve: innervates innervates pectoralis major and pectoralis minor muscles
- Lateral pectoral nerve: innervates pectoralis major muscle
- Intercostobrachial nerve
- Lateral cutaneous branch of second intercostal nerve
- Sensation to medial arm and axilla
- Most common injured nerve with modified radical mastectomy (MRM) or axillary lymph node dissection (ALND)
- Long thoracic nerve
- Arterial supply: branches of
- Internal thoracic (mammary) artery
- Intercostal arteries
- Thoracoacromial artery
- Lateral thoracic artery
- Batson’s plexus: valveless vein plexus, allows direct hematogenous metastasis of breast cancer to spine
- Costoclavicular ligament (Halsted ligament): defines axilla apex
- Breast development
- Formed from ectoderm milk streak
- Hormone influence
- Estrogen: duct development (double layer of columnar cells)
- Progesterone: lobular development
- Prolactin: synergizes estrogen and progesterone
- Cyclic changes
- Estrogen → increased breast swelling, growth of glandular tissue
- Progesterone → increased maturation of glandular tissue, withdrawal causes menses
- FSH, LH surge → ovum release
- After menopause, less estrogen and progesterone results in atrophy of breast and vulvar tissue
- Microscopic anatomy
- Three tissue types
- Glandular epithelium
- Branching system of ducts arranged in a radial pattern extending from the nipple-areolar complex
- Each major duct has branches and ultimately ends in terminal ductules or acini (acini are milk-forming glands of lactating breasts)
- Fibrous stroma and supporting tissues
- Adipose tissue
- Glandular epithelium
- Basement membrane
- Contains laminin, type IV collagen, proteoglycans
- Differentiates in situ from invasive breast cancer
- Three tissue types
Complications
- Metastasis: bone, liver, lungs, brain
Scoring Systems/Classifications
- Gail model → model for assessing breast cancer risk
- Takes into consideration 7 influencing factors
- Doesn’t include genetic factors; may underestimate risk for BRCA1 or BRCA2 mutations and overestimate risk for noncarriers
- Shouldn’t be used in patients with diagnosis of LCIS or DCIS
- Not accurate for African Americans
- Many other models have been created for specific patient populations
- Guide recommendations → high risk
- Close surveillance
- Mammography
- Breast MRI (with lifetime risk > 20%)
- Chemoprevention
- ER modulators: tamoxifen, raloxifene
- Aromatase inhibitors
- Bilateral prophylactic mastectomy and/or salpingo-oophorectomy
- Close surveillance
- Couch model → predicts risk for a mutation in BRCA1 gene
Differential Diagnoses
- Breast abscess
- Fat necrosis
- Breast fibroadenoma
Resources
- National Comprehensive Cancer Network. “Breast Cancer,” (2024)
- American Society of Breast Surgeons. “Consensus Guideline on Genetic Testing for Hereditary Breast Cancer,” (2019)
- Annals of Surgical Oncology. “Contralateral Prophylactic Mastectomy Consensus Statement from the American Society of Breast Surgeons,” (2016)