Colorectal cancer is the third most common cancer in men and women in the U.S. Colorectal cancer is further divided into colon cancer and rectal cancer. The former will be the focus of this page. M. Pillore is credited with creating the first successful colostomy in 1776 for a patient with a malignant rectal tumor. Fine, in 1797, created the first transverse colostomy. Laparoscopic colon resection was first reported by Jacobs in 1991.
Etiology
- The majority of sporadic CRC arises over the course of 10 years from a precursor dysplastic adenoma, involving the following molecular pathway events (60%)
- Early APC (adenomatous polyposis) gene mutations
- Activating mutation in KRAS oncogene
- Inactivation of tumor suppressor gene TP53
- Another major pathway involves CpG island methylator phenotype cancer (35%)
- BRAF gene mutation is is the most common initiating mutation
- Results in inhibition of normal colon cell apoptosis
- Lynch syndrome (5%)
- Risk factors: smoking, alcohol consumption
Pathogenesis
- Transformation of normal colonic epithelium to precancerous lesion and ultimately invasive carcinoma requires an accumulation of genetic mutations
- CRCs often arise from adenomatous polyps that acquire dysplastic changes over the course of 10 – 15 years before actually developing an invasive carcinoma
History
- Usually present with history of abnormal diagnostic colonoscopy
- Asymptomatic
- Diagnostic colonoscopy triggers
- Blood per rectum
- Fatigue
- Abdominal pain
- Anemia
- Changes in bowel habits
- Obstructive symptoms
- Obstructing colon cancer symptoms
- Pencil-thin stools
- Constipation
- Increasingly distended abdomen
- Obstipation
- Abdominal pain
- Vomiting, may be feculent
Labs
- CBC
- LFTs
- CMP
- CEA (carcinoembryonic antigen) levels
- Obtained in order to establish baseline for future comparison
- Serves as a prognostic value for recurrence and survival
- Should not be used for diagnosis or screening
- Genetic testing: MSI, KRAS, BRAF, NRAS
Imaging
- Colonoscopy → most common way of presenting
- Barium enema
- CT colonography
- CT chest, abdomen, pelvis with oral and IV contrast OR noncontrast CT of chest + abdominal MRI are recommended for colon cancer staging (PET/CT or noncontrast chest CT with an MRI of abdomen and pelvis are recommended in patients with allergy or contraindication to iodine contrast)
- Screening
- Begins age 45 in normal-risk adults
- Begins age 40 (or 10 years younger than earliest diagnosis in family, whichever comes first) in adults with intermediate risk (first degree relative with colon cancer diagnosed at age < 60 or two second-degree relatives diagnosed at any age); repeated every 5 years
- Options
- Colonoscopy q10y
- CT colonography q5y
- Flexible sigmoidoscopy q5y
- mt-sDNA stool tests q3y
- Fecal immunochemical (FIT) or guaiac (gFOBT) stool tests q1y
Staging
- Once a patient’s stage is determined, it can be used to guide information regarding survival, treatment, chance of cure, likelihood of residual disease, and recurrence
- Clinical staging → CT chest, abdomen, pelvis with oral and IV contrast OR noncontrast CT of chest + abdominal MRI
- PET/CT isn’t recommended for routine staging, but may be useful in surgical decision making for patients with stage IV disease
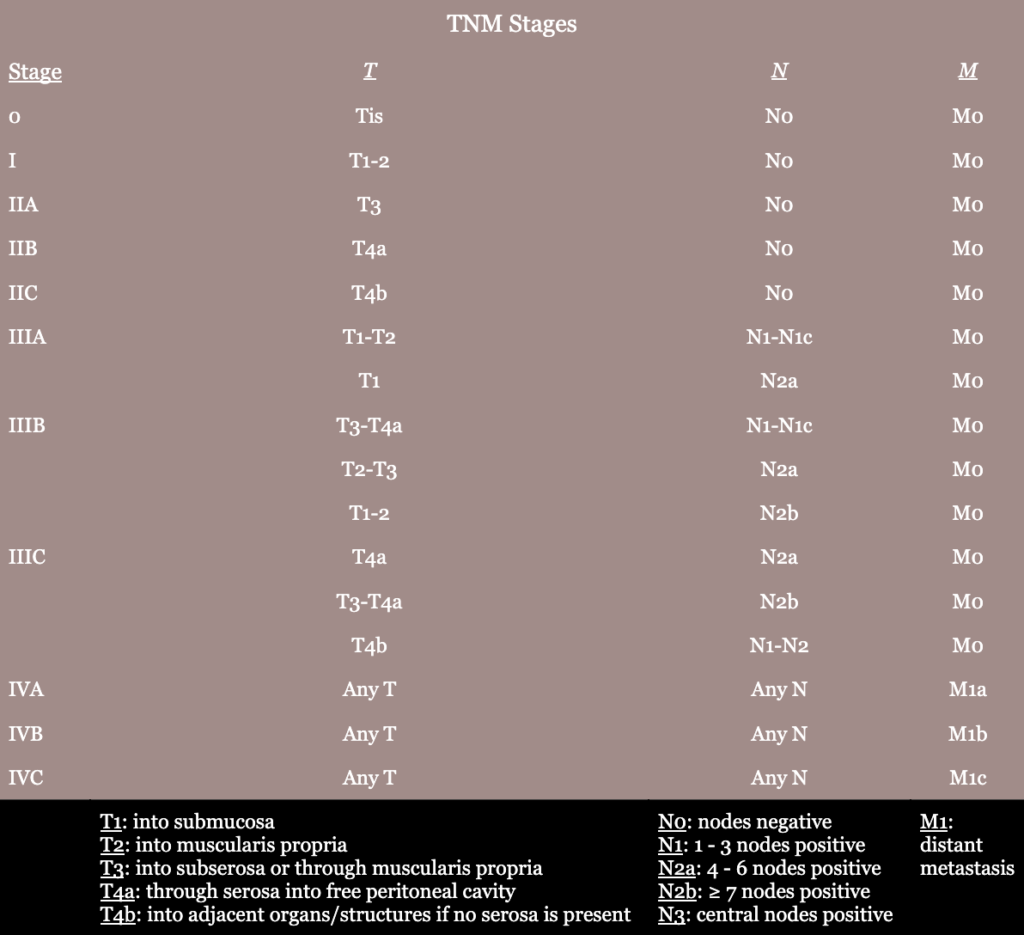
- cTNM (clinical staging): based on evidence obtained from medical history, physical exam, endoscopy, and imaging
- Pathologic staging → should be completed according to the American Joint Committee on Cancer, Tumor, Node, Metastasis (AJCC/TNM) system
- TNM (tumor, node, metastasis) system
- T stage: depth of penetration of tumor into bowel wall
- N stage: extent of lymph node involvement
- M stage: presence or absence of distant metastasis
- TNM (tumor, node, metastasis) system

- Prognostic factors
- CEA level
- Presence of tumor deposits within lymph drainage and their association with blood vessels and neural structures
- Pathology
- pTNM (pathologic examination of the resected specimen)
- ypTNM (pathologic examination of the resected specimen in patients given neoadjuvant therapy prior to resection)
- Histologic grade (low-grade or high-grade) is determined by the pathologist
- Histologic subtype
- Historic staging systems: Dukes classification, modified Astler-Coller classification
Treatment
- Before colectomy, histologic confirmation of invasive adenocarcinoma should be obtained, and the entire colorectal mucosa should be evaluated for synchronous pathology when possible
- The goal of curative surgery in CRC is resection of the primary tumor with adequate free margins, en bloc with loco regional lymphadenopathy
- Free margins
- Free margins help minimize risk of recurrent cancer caused by distal spread and avoids leaving perivisceral lymph nodes, which could be involved in metastatic disease
- 5 cm “free” margin is recommended for colon cancer
- 2 cm distal margin is needed for rectal cancer
- 1 cm margin is used for ultradistal sphincter-sparing surgery
- Free margin at frozen section examination may be acceptable in selected cases of ultradistal sphincter-sparing surgery
- Lymphadenectomy
- Completed to ensure adequate pathologic staging and to remove any possible residual lymph node metastasis
- Need ≥ 12 lymph nodes for proper staging (usually obtain ≥ 20)
- Free margins
- Surgical technique for colon cancer
- Laparoscopic is preferred over open techniques
- Right-sided tumors (cecum and ascending colon) → right hemicolectomy
- Transverse colon tumors → right extended colectomy (middle colic vessels divided at their origin at the level of the inferior margin of the pancreatic neck)
- Splenic flexure tumors → extended right hemicolectomy with ileal-descending anastomosis, extended resection encompassing the splenic flexure, resection of splenic flexure alone
- Left-sided tumors → left hemicolectomy
- Synchronous tumors → two segmental resections or subtotal colectomy
- Surgical treatment for obstructing colon cancer
- Relief of obstruction
- Resection of ischemic/nonviable bowel
- Resection of tumor
- Left-sided obstructions
- Primary anastomosis has historically been avoided – distal stump is closed and proximal stoma is exteriorized (Hartmann operation). However, evidence does support the option of a primary anastomosis in appropriate patients who are hemodynamically stable.
- Sigmoid and left colon obstruction → urgent surgery with segmental resection of primary tumor
- Proximal large bowel perforation or ischemia → subtotal colectomy
- Right-sided obstructions → oncologic segmental resection with either primary ileocolic anastomosis or diverting stoma
- Follow-up colonoscopy at 1 year (check mainly for new primary colon cancer) followed by every 3 years if no polyps are found
- Chemotherapy
- Stage III colon cancer → postop chemo, no XRT
- Stage IV colon cancer, potentially resectable (distant metastases) → preop chemo, restage, and postop chemo, no XRT
- Unresectable colon cancer → chemo ONLY
Relevant Information
- Colorectal polyps
- Protrusion of tissue into the lumen above the surrounding intestinal mucosa
- Usually asymptomatic, but can bleed or cause obstructive symptoms when large
- Some are precursors to cancer
- Characterized according to endoscopic appearance
- Pedunculated: with a stalk
- Sessile: flat
- Can be further classified according to histologic appearance once excised or biopsied → adenoma, hamartoma, inflammatory, serrated, etc.


- Malignancy risk increases dependent on
- Size (large)
- Gross shape (sessile)
- Histologic type (villous)
- Grade of dysplasia
- Polyp excision
- Endoscopic excision is done for adenomatous polyps
- If there are large polyps that can’t be removed endoscopically, then surgical intervention is considered
- Method
- Pedunculated polyps → cold or hot snare polypectomy
- Sessile polyps → elevated with saline injection and excised with variety of techniques
- Sufficient endoscopic excision criteria
- T1
- 2 mm margins
- Well differentiated
- No vascular or lymphatic invasion
- Repeat colonoscopy guidelines
- U.S. Multi-Society Task Force on Colorectal Cancer. “Recommendations for Follow-Up After Colonoscopy and Polypectomy,” (2020)
- European Society of Gastrointestinal Endoscopy. “Post-Polypectomy Colonoscopy Surveillance,” (2020)
- Important anatomic vascular landmarks
- Ileocolic pedicle originates from superior mesenteric vessels just caudal to the second portion of the duodenum
- The middle colic vessels originate from the superior mesenteric vessels at the level of the inferior margin of the pancreas
- The inferior mesenteric vein can be easily identified at the level of the ligament of Treitz
- The IMA originates from the aorta, 2 – 3 cm caudal from the area where the IMV is identified; its origin is surrounded by the mesenteric and hypogastric nervous plexus
- The left colic artery originates around 2 cm distal to the origin of the IMA
- The vascular supply of the colon relies on marginal vessels in the mesocolon; care must be taken during mobilization and manipulation of the mesocolon since minimal injury of these vessels can lead to irreversible ischemic damage
Complications
- Metastasis: liver (most common), lung, ovary
- Death
Scoring Systems/Classifications
- Haggitt classification
- Classifies depth of invasion for pedunculated malignant polyps
- Level 0: carcinoma limited to the mucosa, carcinoma in-situ
- Level 1: carcinoma invading into submucosa, limited to head of the polyp
- Level 2: carcinoma invading level of neck (junction of head and stalk)
- Level 3: carcinoma invading any part of the stalk
- Level 4: carcinoma invading into submucosa of colon wall, below level of stalk but above muscularis propria; sessile polyps with invasion of muscularis mucosa
- Kikuchi classification
- Classifies depth of invasion into submucosa for sessile malignant polyps
- Sm1: invasion into upper third of the submucosa
- Sm2: invasion into the middle third of the submucosa
- Sm3: invasion into the lower third of the submucosa
Differential Diagnoses
- Carcinoid tumors
- Crohn disease
- Gastrointestinal lymphoma
- Ischemic bowel
- Ileus
- Small intestine carcinoma
- Small intestine diverticulosis
- Ulcerative colitis
Resources
- American Society of Colon and Rectal Surgeons. “Clinical Practice Guidelines for Management of Colon Cancer,” (2022)